1. Product indication introduction
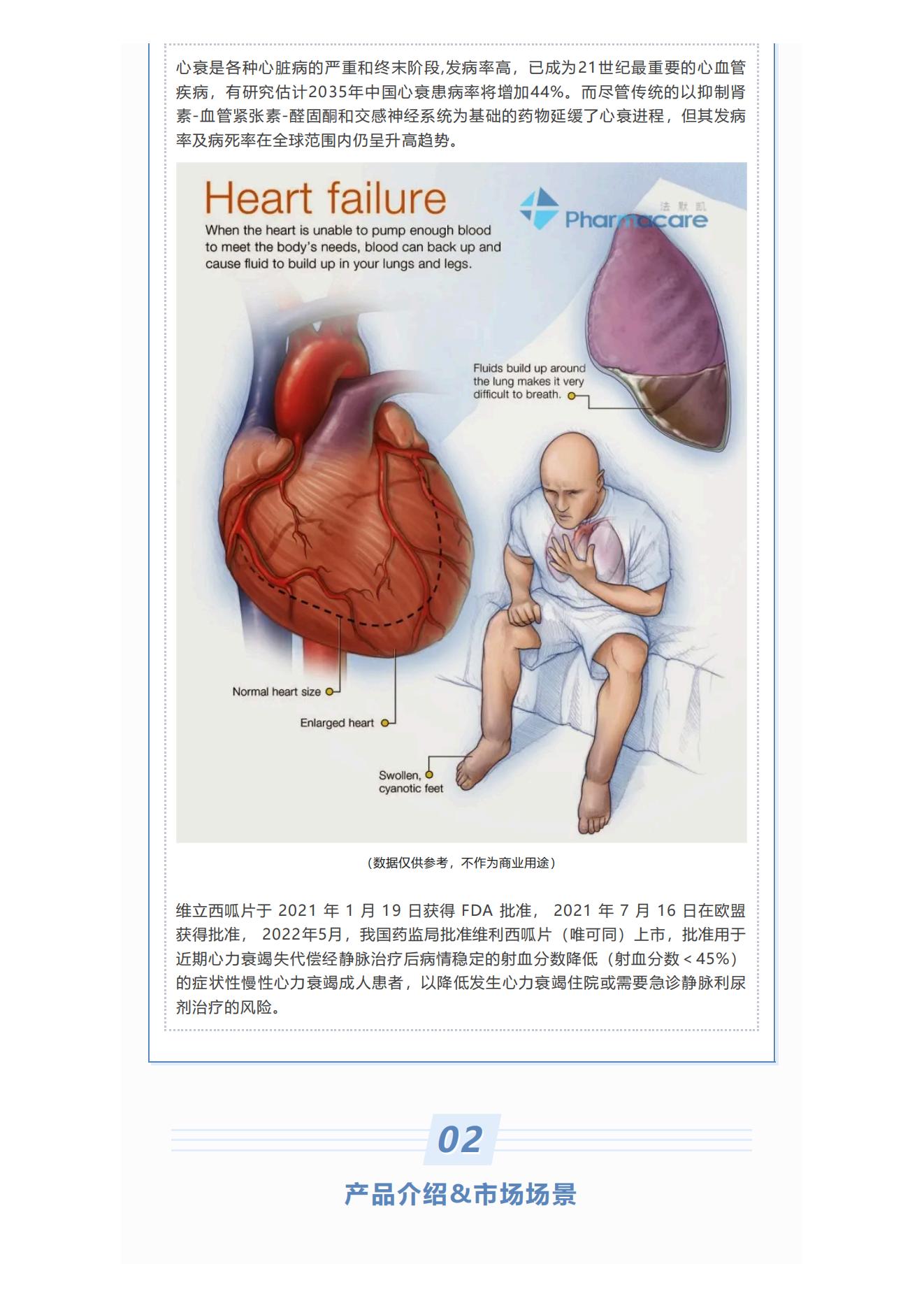
Heart failure (heart failure)
Heart failure is a serious and end-stage of various heart diseases with a high incidence. It has become the most important cardiovascular disease in the 21st century. Studies estimate that the prevalence of heart failure in China will increase by 44% in 2035. Although traditional drugs based on inhibiting the renin-angiotensin-aldosterone and sympathetic nervous systems have slowed down the progression of heart failure, its morbidity and mortality are still on the rise globally.
Vericiguat tablets were approved by the FDA on January 19, 2021, and obtained approval in the European Union on July 16, 2021. In May 2022, the Chinese drug regulatory agency approved the marketing of vericiguat tablets (Verquvo®) for symptomatic chronic heart failure adult patients with reduced ejection fraction (ejection fraction <45%) who are stable after intravenous treatment for recent decompensated heart failure, to reduce the risk of hospitalization for heart failure or the need for emergency intravenous diuretic treatment.
2. Product introduction & market scenario
Product introduction:
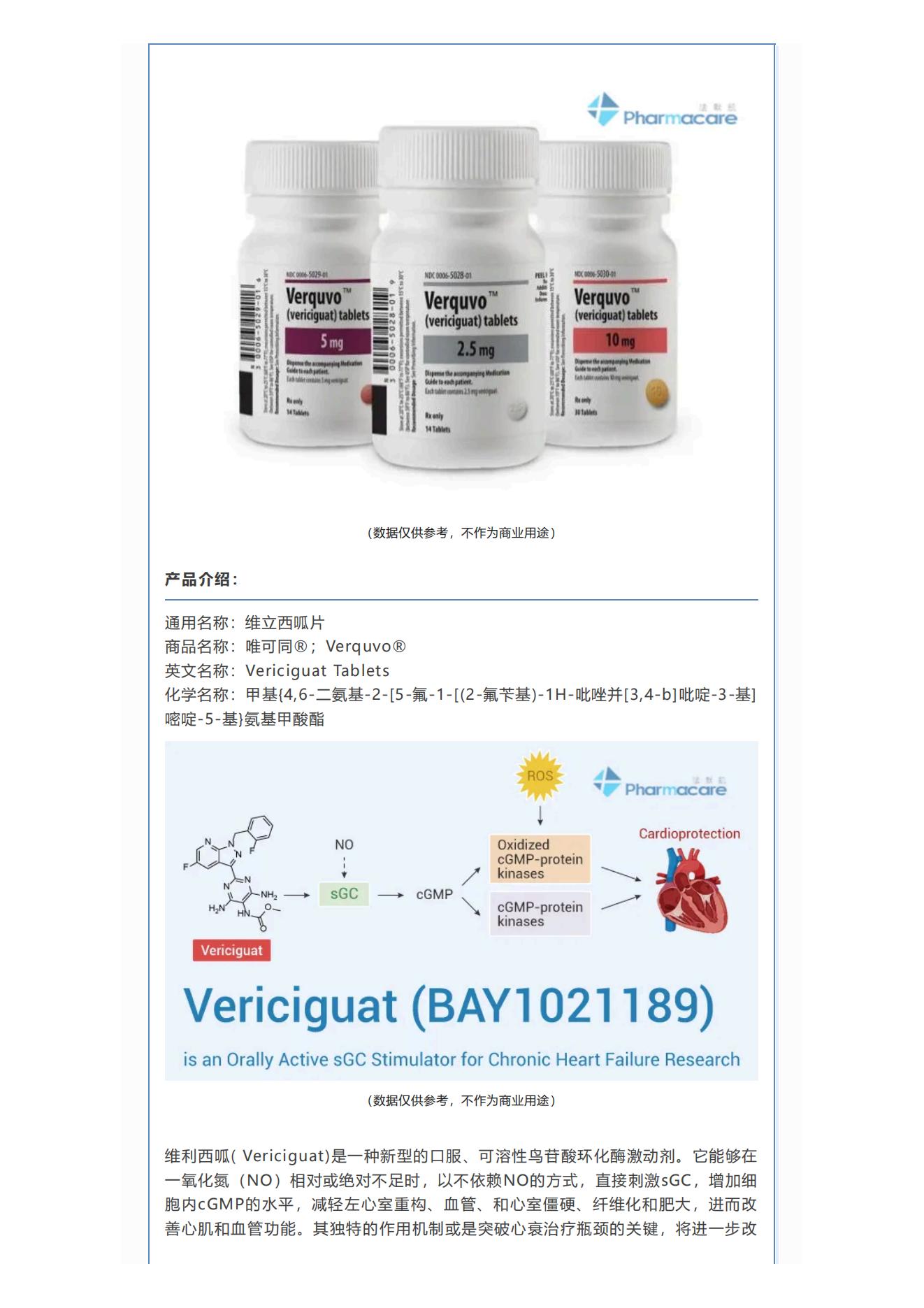
Generic name: Vericiguat Tablets
Brand name: Verquvo®; Verquvo®
English name: Vericiguat Tablets
Chemical name: Methyl {4,6-diamino-2-[5-fluoro-1-[(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]pyrimidin-5-yl}carbamate
Vericiguat is a new type of oral, soluble guanylate cyclase activator. When nitric oxide (NO) is relatively or absolutely insufficient, it can directly stimulate sGC in a NO-independent manner, increase the level of intracellular cGMP, reduce left ventricular remodeling, vascular and ventricular stiffness, fibrosis and hypertrophy, and then improve myocardial and vascular function. Its unique mechanism of action may be the key to breaking through the bottleneck of heart failure treatment. It will further improve the prognosis of patients with heart failure. It is currently the only heart failure drug targeting cell-signaling pathways. Therefore, the listing of vericiguat provides a new treatment option for adult patients with symptomatic chronic heart failure.
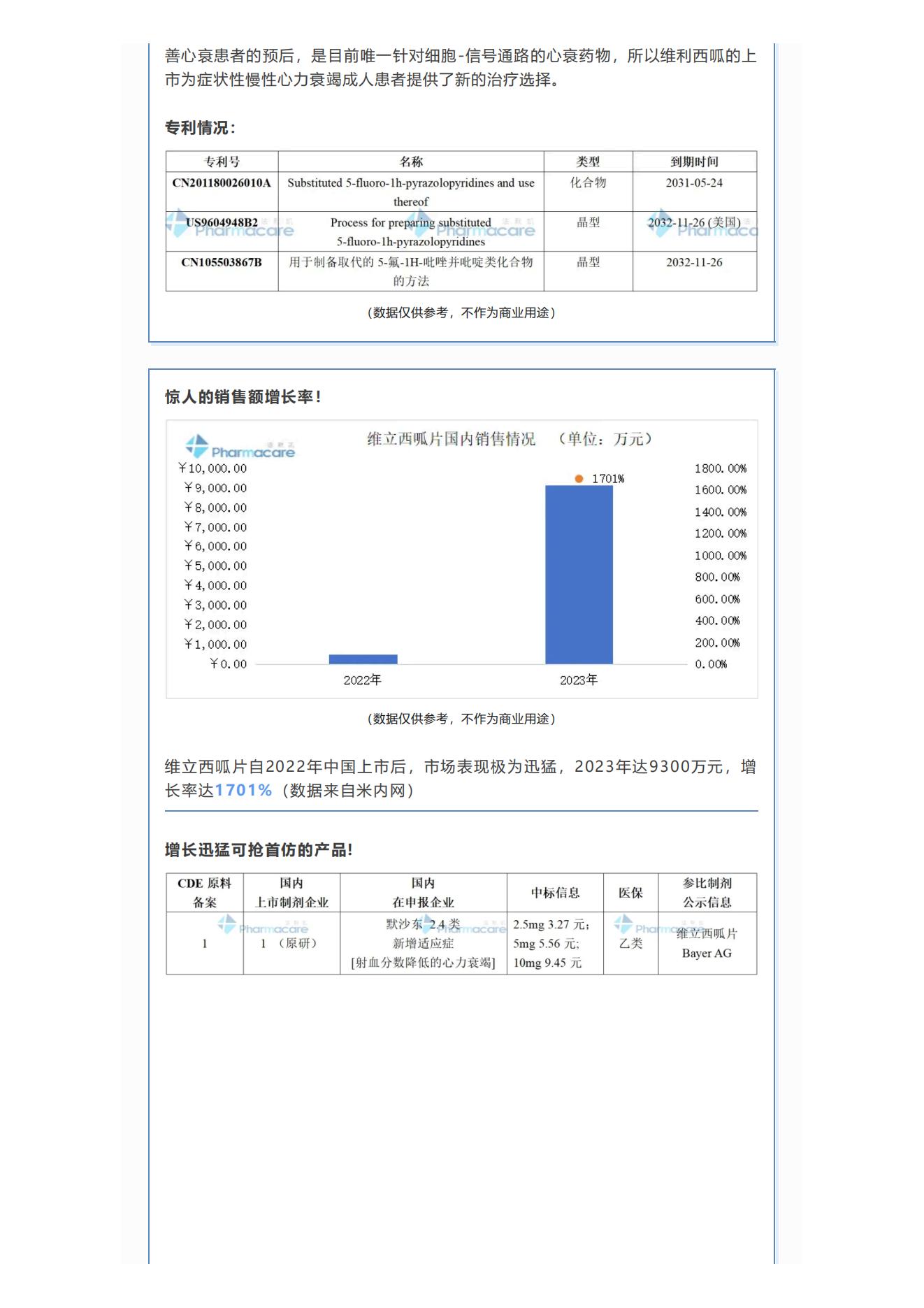
Since the listing of vericiguat tablets in China in 2022, the market performance has been extremely rapid. In 2023, it reached 93 million yuan, with a growth rate of 1701% (data from Menet).
Cardiovascular system drugs mainly include antihypertensive drugs, heart disease treatment drugs, blood lipid drugs and cerebrovascular treatment drugs. Among them, heart disease treatment drugs account for about 30% of the total cardiovascular market, with a scale of more than 20 billion.
For the current comparison and analysis of the main market competitiveness and market growth rate of products in the same ATC category, according to Pharmaceutical Cube data, vericiguat is currently a product with rapid growth and no competition. However, after the patent period expires and multiple generic drugs are listed, it will also face fierce market competition in the future. Therefore, the progress and cost control of the first generic version of vericiguat preparations are particularly crucial.
3. Competitiveness and advantages
Product competitiveness:
The 2021 ESC heart failure guidelines included vericiguat for the treatment of heart failure with reduced ejection fraction (HFrEF) for the first time. It is recommended to use vericiguat for patients with NYHA cardiac function grades II-IV. On the basis of applying ACEI (or ARNI), beta blockers and MRA, combination therapy is still recommended for patients with worsening heart failure to reduce the risk of cardiovascular death or heart failure hospitalization.
The 2022 AHA/ACC heart failure guidelines also included vericiguat in heart failure treatment. In selected high-risk HFrEF patients and those who have received GDMT treatment but have recently worsened (NYHA grades II-IV, LVEF ≤45%, recent hospitalization due to worsening heart failure, use of intravenous diuretics, elevated NT-proBNP), oral soluble guanylate cyclase stimulators (vericiguat) can be considered to reduce the hospitalization rate and cardiovascular mortality of patients with heart failure.
The advent of vericiguat marks a new era in heart failure treatment. Its unique mechanism of action, wide applicability in different populations, significant effects in clinical trials, and advantages such as successful inclusion in the medical insurance catalog make it a new treatment option for patients with heart failure.
Pharmacare raw material advantages:
Professional cardiovascular API manufacturer in India. The raw materials can support the application for preparations in China, the United States and Europe.
High quality standard, good impurity control, large batch size.
Competitive price, long validity period of API, large production capacity, exported to many countries and regions around the world.
Flexible and convenient delivery, regular inventory after product approval.
CDE filing of raw materials.
For cooperation and negotiation, please contact:
Manager Chen: 18888123663 (same as WeChat)
Manager Chen: 13356653012 (same as WeChat).
Reference materials:
1. IQVIA
2. Menet data
3. Pharmaceutical Cube data
4. Insight data