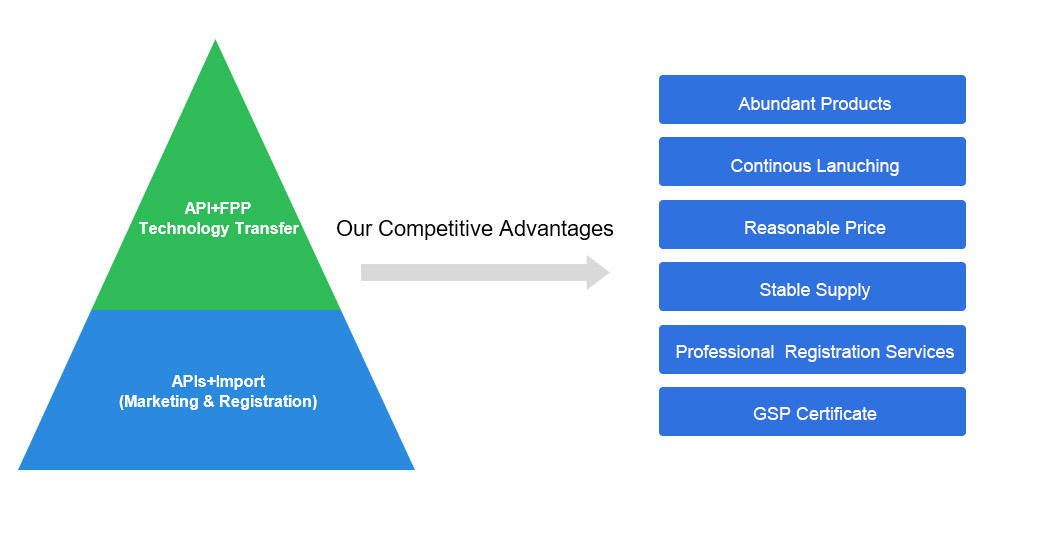
Business model and Advantages
APIs that have obtained China's import license or DMF have been filed: direct procurement, which can increase a second supplier or directly use for formulation research and development, with low risk.
APIs planned for Chinese DMF filing: All APIs planned for Chinese DMF filing have completed USDMF filing to meet the Chinese registration requirements, such as detailed impurity studies, genotoxic impurities research, etc. Domestic formulation customers can be associated, provide impurity reference substances, etc., to shorten the customer’s R&D cycle
APIs for commercial production: to ensure global supply, competitive price advantage, joint formulation development, and provide necessary support (reference substance, DMF filing, etc.)
NCE: The technical difficulty is relatively high, and the patent needs challenges. Efficient R&D plan and strategic cooperation with customers in advance to seize the first imitation opportunity
