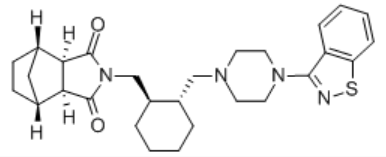
Specification:20mg,40mg,60mg,80mg (Formulation strength)
Indications:
1.Treatment of adult and adolescent patients (13 to 17 years) with schizophrenia
2.Monotherapy treatment of adult and pediatric patients (10 to 17 years) with major depressive episode associated with bipolar I disorder (bipolar depression)
3.Adjunctive treatment with lithium or valproate in adult patients with major depressive episode associated with bipolar I disorder (bipolar depression)
Origin:India
Batch Size:>59kg
Registration status:
FDA:27570 A
CDE: Y20200000744 I
Advantage:
The formulation which used the same API has TA in US FDA, we can offer formulation Technology Transfer service.
Exclusive API, independent review in CDE