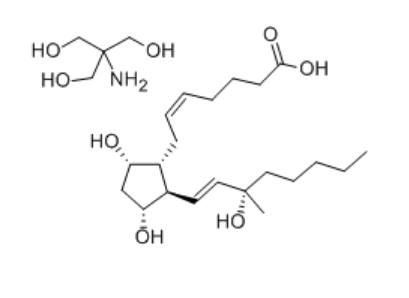
Specification:250ug (Formulation strength)
Composition:
Description:
Storage:
Indications:
1.Failure of expulsion of the fetus during the course of treatment by another method; 2. Premature rupture of membranes in intrauterine methods with loss of drug and insufficient or absent uterine activity; 3. Requirement of a repeat intrauterine instillation of drug for expulsion of the fetus; 4. Inadvertent or spontaneous rupture of membranes in the presence of a previable fetus and absence of adequate activity for expulsion
Pharmacopeia:
DMF:
GMP:
Origin:India
Batch Size:100g
Registration status:
FDA:27865 A
EU:√
CDE: Y20200000103 I
Advantage:
The only approved API used in US listed generic drugs
Exclusive API, independent review in CDE